About LifeTec Group
LifeTec Group is a pre-clinical contract research organisation. We aim to support you in translating succesful results from bench to in-vivo, by focusing on the relevance of realistic environments rather than standardization. Our people will act as an extension of your own team, where LifeTec Group will focus on a realistic testing scenario and your team can keep their focus on device or therapy development.
Our team is ready to help!

The LifeTec Group approach
LifeTec Group's mission is to support good ideas with creative preclinical services and life mimicking enabling technology to turn them into great medical innovations.
Our approach is to focus foremost on making a concept work, and then plot out the appropriate testing strategy. We have seen many new concepts look good in the early stages, and then fail in-vivo. And although in-vivo is the golden standard and absolutely necessary, if your device concept doesn't perform as needed, it is often very hard to determine the cause for it. LifeTec Group therefore focuses on re-creating the in-vivo situation, but in a laboratory setting where we can actually demonstrate and show what works or why something fails. And with that information, success is more likely in the end.
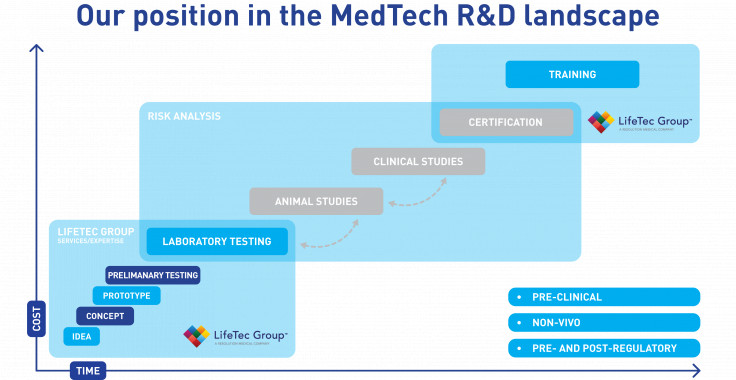
The R&D Roadmap
The pathway to the patient
In the R&D pathway that leads to clinical use of innovative device concepts there are multiple stages, from early-stage concept to clinical product. As the project matures, the regulatory system directs studies along well-known and standardized testing methods. In an early stage, laboratory bench test models and associated protocols are used to verify functionality and durability of devices. With good results, the next phase involves animal studies to demonstrate that the procedures are feasible and that the animal responds well over prolonged periods to the successful treatments. In the final stages, human clinical studies provide final proof that the device innovation and procedures are feasible, safe, reliable and beneficial to the health of the patients.
Shorten R&D timeline with smart approach
These three subsequent stages are time consuming and costly, and therefore device or procedure iterations that late on the R&D pathway should be avoided. It is therefore of paramount importance to have a well-considered test and development plan that looks forward. For example, standardized testing on benchtop equipment is hugely important, but well-established test methods are not always representative for in-vivo situations - especially for innovative device concepts. In LifeTec Group, we develop test methods that are realistic and representative for the in-vivo animal or human situations to ensure that an innovative concept works. In that way the device concept is developed in a relevant situation and a smooth transition to in-vivo can be expected, ultimately shortening your R&D timeline
Throughout the three major stages described above, the device itself does not change. However, the environment in which the device is assessed changes significantly from one stage to the next, and as a result these transitions may become significant hurdles if the results are not as good as expected from a previous stage's outcomes.
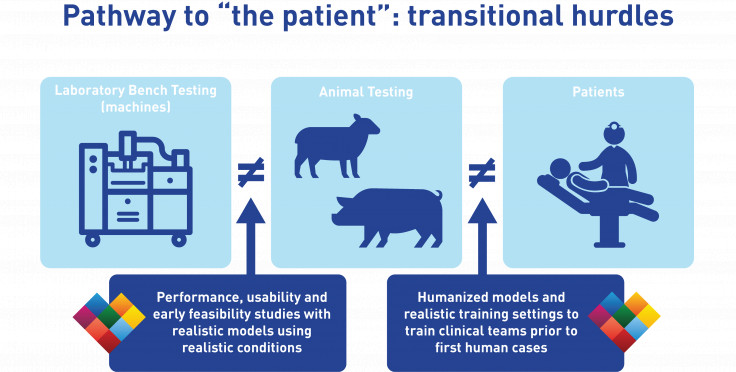
Transitional hurdles
On the path from bench to animal to patient studies, the device itself does not change as its design is frozen. However, the environment in which the device is assessed changes significantly from one stage to the next, and as a result these transitions in test environment may become significant hurdles if the results are not as good as expected from a previous stage's outcomes. Young and healthy animal tissue responds differently than mechanical components from a standardized test equipment, but also different from tissue from elderly and often pathological patients. Moreover, procedural feasibility combined with functional assessment is strongly depending on anatomical features, which differ between animals and humans and are very hard to accurately capture in standardized test equipment. As a result from these differences in environment, it can happen that early bench tests look promising but in-vivo studies disappoint.
Realistic BioSimulators for R&D and clinical training
LifeTec Group aims to shorten your R&D path by taking a smart approach: by making use of realistic and relevant tests available in the laboratory testing stage, you will be well prepared for the following steps. LifeTec Group's BioSimulator platforms are developed to replicate the in-vivo animal or human environment, allowing to ensure the feasibility of your procedure before commencing in-vivo work. In this approach, you already know you can do the procedure in an early stage, making you well-prepared to do the in-vivo studies to find out long-term response on the intervention and clinical safety. In this approach we can smooth your transition from bench to animal, reducing the need for some of the early acute animal studies and shortening your timeline.
Our BioSimulator platforms are also versatile in configuration, meaning that we can adapt our models to human anatomies and clinical imaging. In that approach, our platforms have been very useful in procedural training of clinical teams - both on the intervention and on the imaging - so that these teams have had a very realistic training experience prior to their first human cases. This provides a great benefit for the first study outcomes, and not in the least for the patients.
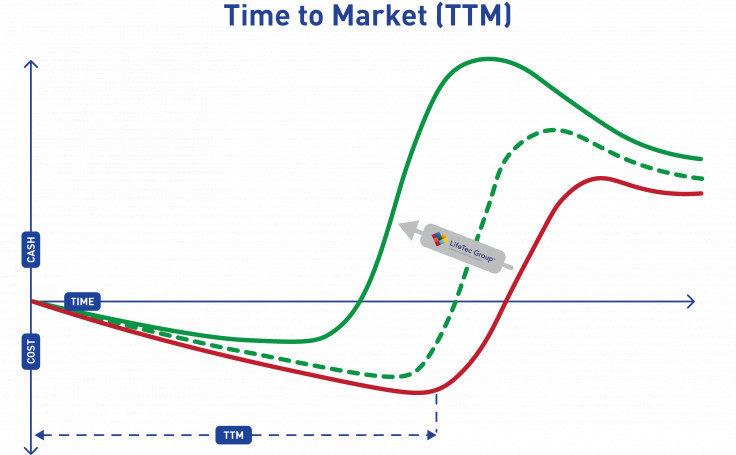
Shorten Time-to-Market
By using a smart approach throughout your development program, you will save time and expenses. This can result in an earlier design freeze and earlier market introduction at lower cost, and will give you a headstart on competitors leading to a higher initial market share.
LifeTec Group focuses strongly on creating relevant and realistic test and assessment approaches that help you achieve these goals. By working together, LifeTec Group provides these clinically relevant (bio-)simulation environments at an early stage and your team can fully focus on the development of the products and the clinical training program to ensure that your innovation can perform as expected and your end-users know how.
Interested in our BioSimulator Systems?
Would you like to know more about our BioSimulator Systems? Read more on the BioSimulators pages.
Curious about how you can benefit?
For more information on our services, how you can have access to our BioSimulators and make use of our experienced Team, read more on our Services section.
Download our factsheet
For more information on how to work with LifeTec Group, please download our factsheet!
Interested in more about what we do at LifeTec Group? Contact us!
Call at +31 40 2989393 Or e-mail us
