Recently, our partner CoreMedic announced the first successful implantation of their novel transcatheter device for mitral valve repair. Their ‘ChordArt’ device is used to treat primary regurgitation in the mitral valve, where the valve suffers from a flailing leaflet, which is currently one of the most common heart valve diseases worldwide. We are proud to be part of their journey towards this huge milestone.
LifeTec its Cardiac BioSimulator has enabled CoreMedic to evaluate every new iteration of the ChordArt device throughout the development process in a clinically realistic setting, resulting in a time and cost-effective process towards their first successful clinical case. The Cardiac BioSimulator was set up with an Inferior Vena Cava-mimic to simulate groin entrance for the ChordArt device. By guiding the device through the atrial septum via a transseptal puncture, the approach to the mitral valve in the simulator is essentially clinically relevant; steering angles and distances are fully realistic and therefore very well comparable with the eventual clinical cases.

By using real hearts, the haptic feedback from device-tissue interaction is more comparable to the clinical situation compared to alternatives like silicon, 3D-printed or digital models. Additionally, since real hearts are used in the Cardiac BioSimulator, a completely realistic anatomy of the mitral valve, left atrium and chordal system is present during these tests. Depending on the required level of realism, either porcine slaughterhouse hearts or human cadaver hearts were used during this development-focused partnership to always have the most suitable setting available for every single test.
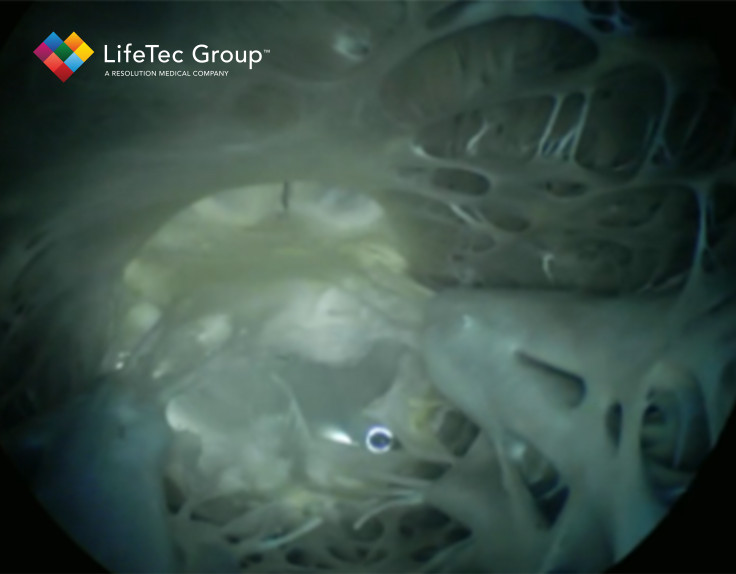
The Cardiac BioSimulator is completely compatible with clinical imaging techniques such as transesophageal ultrasound visualization, transgastric ultrasound visualization and even proper fluoroscopy views. This ensures that also the visualization of the device inside of the heart during a session in the Cardiac BioSimulator is completely relevant according to clinical standards, with proper views of the atrial septum, mitral valve, papillary muscles and the ChordArt device in this specific example. Those ultrasound and fluoroscopy views, combined with the unique capability to include live cardioscopy, makes the imaging options in and around this setup highly valuable and more comprehensive compared to other alternatives like animal or cadaver studies.
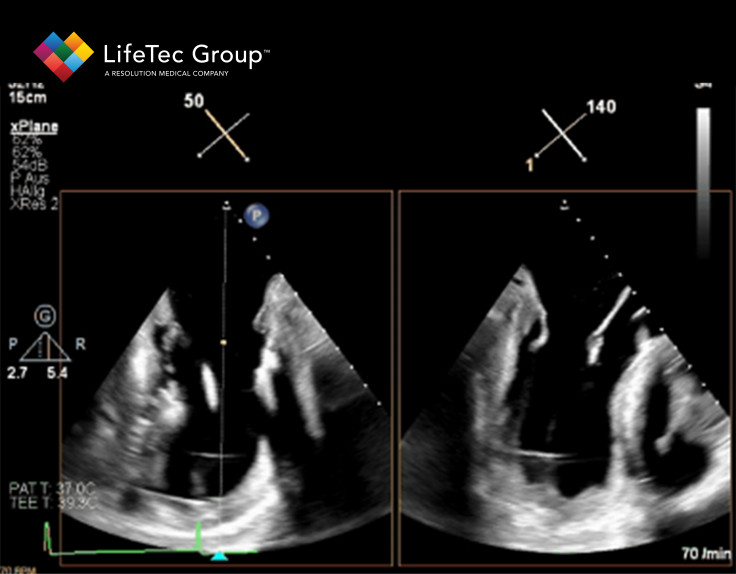
Altogether, by combining a realistic device trajectory with real hearts and proper, relevant and unique imaging views, LifeTec its Cardiac BioSimulator was one of the drivers behind the efficient development process that CoreMedic went through when developing their ChordArt device. This was all achieved without the need for excessive animal testing, while still maintaining a highly realistic setting throughout the whole process, which are aspects that LifeTec Group is pursuing at all times.
We highly appreciate our partnership with CoreMedic, with which lots of fruitful discussions always resulted in valuable improvements on all aspects, indicating great teamwork and collaboration. Via this way, we want to congratulate the whole CoreMedic team on this milestone and we look very much forward to extend our collaboration in the future.
Interested to find out how our Cardiac BioSimulator can help you during the developmental process of your new cardiovascular device? Or curious to learn how we can support physician-training for your existing device with this setup? Feel free to reach out to our R&D team.
Interested in more about what we do at LifeTec Group? Contact us!
Call at +31 40 2989393 Or e-mail us
