Paper
"Cartilage Regeneration in Preannealed Silk Elastin-Like Co-Recombinamers Injectable Hydrogel Embedded with Mature Chondrocytes in an Ex Vivo Culture Platform"
Although the use of hydrogel is the most promising biomaterial, it often lacks in rigidity and anchorage of cells when they are surrounded by synovial fluid while they are subjected to heavy loads.

Authors
Filippo Cipriani | Melanie Krüger | Israel Gonzalez de Torre | Luis Quintanilla Sierra | Matilde Alonso Rodrigo | Linda Kock | José Carlos Rodriguez-Cabello
Purpose of this study
This study focuses on the correlation between the elastin motifs and silk motifs, in order to understand how to improve the gelation properties of the hydrogel to obtain a system capable of forming an ECM fibrillary structure directly after injection.
Abstract
Tissue engineering for cartilage repair requires biomaterials that show rapid gelation and adequate mechanical properties. Although the use of hydrogel is the most promising biomaterial, it often lacks in rigidity and anchorage of cells when they are surrounded by synovial fluid while they are subjected to heavy loads.
We developed and produced the Silk Elastin-Like co-Recombinamer (SELR), which contains both the physical interaction from elastin motifs and from silk motifs.
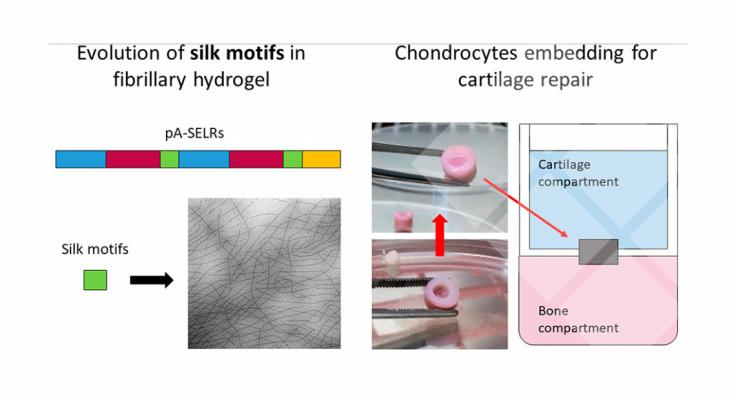
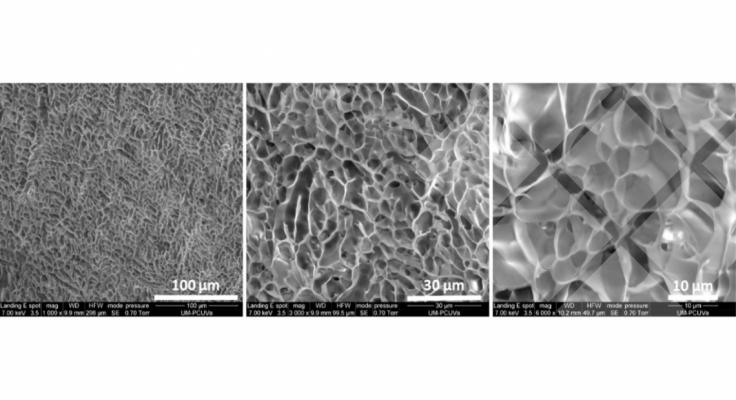
In the first part of this work, we set up and optimized a preannealing treatment based on the evolution of silk motifs into β-sheet structures in order to fulfill the required mechanical properties of hydrogels for cartilage repair. The new preannealed SELRs (pA(EIS)2-(I5R)6) were characterized with the combination of several experimental techniques (CD, TEM, SEM, and rheology) to provide a deep insight into the material features.
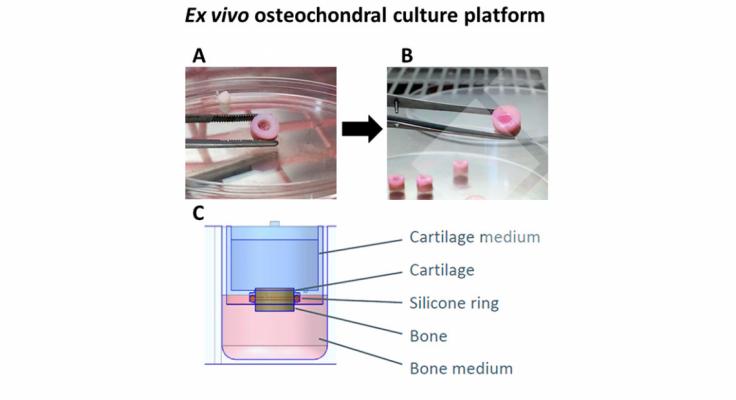
Finally, the regeneration properties of the pA(EIS)2-(I5R)6 hydrogel embedded with chondrocytes were evaluated. After 4 weeks of culturing in a standardized and representative ex vivo model, the biochemical and histological analysis revealed the production of glycosaminglycans and collagen. Moreover, the immunohistochemistry showed the absence of fibro-cartilage and the presence of hyaline cartilage. Hence, we conclude that the pA(EIS)2-(I5R)6 hydrogel presents improved mechanical properties while conserving the injectability, which leads to successful regeneration of hyaline cartilage in an ex vivo model.
Conclusions
We developed and produced the preannealed Silk Elastin co- Recombinamer (pA(EIS)2-(I5R)6), which shows unique properties as a promising candidate for tissue engineering applications.
We have focused on the need for biomaterials for cartilage repair, capable of being delivered into the area of interest, showing a rapid gelation and adequate mechanical properties when surrounded by synovial fluid.
We have set up and optimized a preannealing treatment based on the evolution of silk motifs into β-sheet structures and on the phenomenon of thermal memory.
In this study, we have carried out the physicalcharacterization of ourmaterial in order to provide adeeper insight into the material features, analyzing the contribution of each component (silk and elastin) for the cross-linking formation.
The pA(EIS)2-(I5R)6 has shown a fast gelation, improved mechanical properties, and the presence of a fibrillary structure directly after injection of the hydrogel. Moreover, culturing the hydrogel embedded with chondrocytes in the ex vivo culture platform for weeks has exhibited good biocompatibility and remarkable advantages; such as the de novo ECM formation, the absence of fibro-cartilage, and the production of hyaline cartilage.
The addition of the silk allows making hydrogels with a lower concentration, leading to larger pores, which is most likely responsible for better cell spreading and proliferation. In conclusion, the pA(EIS)2-(I5R)6 has been shown to have new outstanding properties, which make the hydrogel a promising injectable scaffold in the field of cartilage regeneration.
Download journal publication:
This project has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie grant agreement No. 642687.
The authors are grateful for the funding from the European Commission (NMP-2014-646075), the Spanish Government (PCIN-2015-010, MAT2015-68901-R, MAT2016-78903-R, MAT2016-79435-R), Junta de Castilla y Leoń (VA015U16), and Centro en Red de Medicina Regenerativa y Terapia Celularde Castillay Leoń.
- COLOFON
Marie Sklodowska-Curie grant agreement No. 642687

Interested in more about what we do at LifeTec Group? Contact us!
Call at +31 40 2989393 Or e-mail us
