Journal of Medical Devices
"Feasibility of a Mitral Annuloplasty With the Capability for Peri- and Postoperative Adjustment."
We are proud to report that our team was able to support the University of Cambridge in a research project on an adjustable mitral annuloplasty device that resulted in a publication in ASME's Journal of Medical Devices in June 2020. Jacob Brubert, working for the Department of Chemical Engineering and Biotechnology in Cambridge, reports on an innovative idea to implant a device prototype in the annulus of the mitral valve that allows reshaping of the annulus during and after the implantation of the device! In this project, the team from Cambridge performed their device implant studies in our Cardiac BioSimulator platform and published the results in this paper.

Re-Shapeable
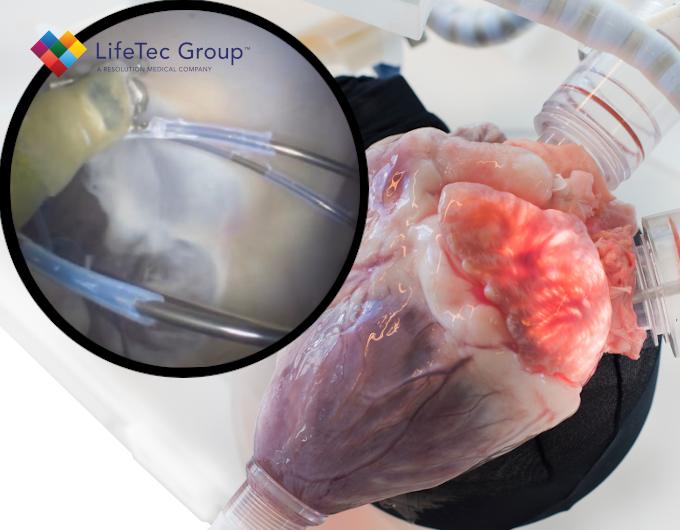
Mitral valve insufficiency is often treated using pre-shaped mitral annuloplasty devices. However, literature suggests that these pre-shaped devices can still be improved on as patient-specific anatomy and pathology can still result in minor valve insufficiency post-operatively, and the re-shaping directly affects the durability and effectiveness of the therapy. As a potential solution, the Cambridge University group has developed a REshapeable Mitral Annuloplasty DevIce (REMADI) that is not fixed to a preset geometry. The new device concept allows for peri-operative adjustment during the implantation procedure, but can also be further optimized post-operatively. As shown in the picture, the ring-shaped device is fitted with single-shot anchors for attachment to the mitral annulus tissue, and is mounted to the vertical wires of a delivery system. These vertical wires can provide a radial force, expanding the annuloplasty device into a c-shaped structure for implantation where each of the vertical wires can be controlled independently. The prototype device material is softened or hardened by an alternating magnetic field to reshape and then set the device into a fixed shape.

Implantation studies
To demonstrate proof of concept for this device prototype, it was important to use a realistic environment in which the delivery of the device could be demonstrated, as well as the anchoring into the tissue, the reshaping of the device ring and the application of the external alternating magnetic field to change the material properties of the ring. Furthermore, it was important to assess (the change in) the functional performance of the mitral valve during and following the reshaping exercise. The Cardiac BioSimulator platform provided exactly that, and the collaboration between the Cambridge University and LifeTec teams was a success just like the results of the study that are presented in the paper.
What's in it for you?
LifeTec Group offers realistic and relevant preclinical assessments of new device innovations in an early stage of development, and brings years of experience in test design to the table. The work presented in the paper above is a typical example of how LifeTec Group can boost your project. Our lab has various types of platforms that focus on clinically relevant experiments, and our team is ready to discuss any customization of protocols that might be needed to create the perfect match to your needs. Usually, we can serve your team with relevant experimental data within a few weeks and this greatly speeds up development time compared to developing your own test method or applying for ethics for the early acute animal studies. If you're interested in generating early results, final validation prior to animal or clinical work, or even training to end-users of your products, feel free to reach out to our team!

The published article:
Jacob Brubert, Steven Tsui, Paul De Sciscio, Geoff D. Moggridge
Feasibility of a Mitral Annuloplasty With the Capability for Peri- and Postoperative Adjustment
Journal of Medical Devices, Jun 2020, 14(2): 025005
Interested in more about what we do at LifeTec Group? Contact us!
Call at +31 40 2989393 Or e-mail us
