Our Cardiac BioSimulator once again contributed to a publication. This publication describes the first ex vivo cardiac study of a biological heart valve prosthesis equipped with intravalvular impedance (IVI) sensing. The study aimed to evaluate the feasibility of IVI sensing for monitoring heart valve prostheses after implantation, specifically when the sensorized valve is surrounded by biological tissue.
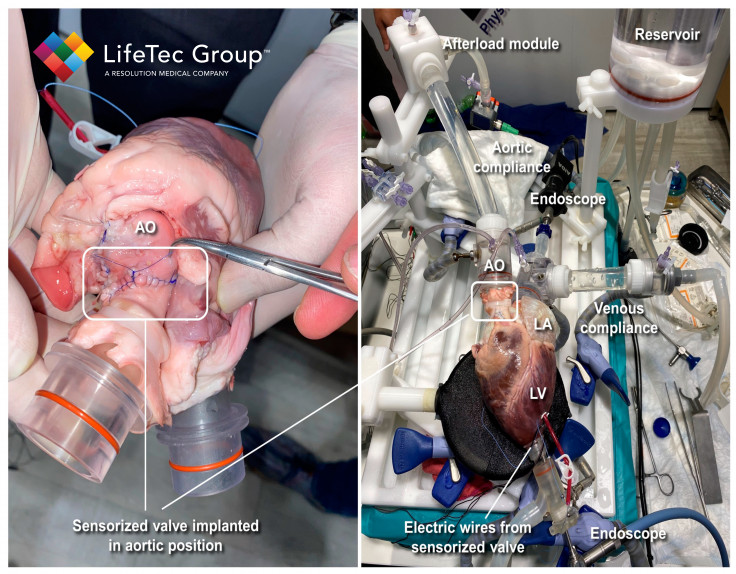
The research was conducted by a team of authors led by Cercenelli, Gironi, Bortolani, and Marcelli. The study used a commercial model of a biological heart valve (Soprano Armonia, LivaNova PCL) and embedded three miniaturized electrodes in the valve leaflets. These electrodes were connected to an external impedance measurement unit. The sensorized valve was then implanted in the aortic position of an explanted porcine heart, which was connected to our Cardiac BioSimulator.
The IVI signal was recorded in different dynamic cardiac conditions replicated by the Cardiac BioSimulator. The study evaluated the maximum percent variation in the IVI signal and calculated its first derivative (dIVI/dt) to assess the rate of valve leaflet opening/closing. The results showed that the IVI signal was detectable when the sensorized valve was surrounded by biological tissue and exhibited similar trends to those observed during in vitro experiments. Additionally, changes in dIVI/dt indicated variations in the rate of valve opening/closing in different dynamic cardiac conditions.
The role of LifeTec Group
The Cardiac BioSimulator study provided by LifeTec Group played a crucial role in the research as it allowed for the realistic simulation of physiological conditions and dynamic cardiac behavior. For this particular study, the platform consisted of an explanted porcine heart connected to a pulsatile fluid dynamic system. It replicated the pulsatile flow through the heart valves along with the associated blood pressures.
The platform also incorporates various components to mimic physiological conditions, such as a compliant silicone tube to simulate aortic compliance, an afterload module to simulate peripheral vessel resistance, and a compliant silicone tube to mimic venous compliance. These elements contributed to creating a realistic environment for evaluating the performance of the sensorized biological heart valve (BHV) under dynamic cardiac conditions.
LifeTec Group's expertise in developing and providing such advanced bioengineering platforms allowed the researchers to conduct ex vivo testing on the sensorized BHV. By utilizing the Cardiac BioSimulator platform, the researchers could evaluate the IntraValvular Impedance (IVI) sensing approach in a setting that closely resembled in vivo conditions.
If you are interested in more, you can download the publication here:
What's in it for you?
LifeTec Group’s Cardiac Biosimulator (CBS) can provide you with a realistic, relevant and fully controllable cardiac simulator environment for R&D or training purposes. Using our biomedical engineering skills to the fullest, we are there to help you during your process of making your concept into a product that is ready for the market. We have a strong belief that (start-up) companies that are aiming to develop a cardiac device or therapy can really benefit from BioSimulations!
Does this sound interesting for you? Do not hesitate to reach out if you want more information about what services we can offer you!
Interested in more about what we do at LifeTec Group? Contact us!
Call at +31 40 2989393 Or e-mail us
