In this study
Nanofibrillar cellulose as a naturally biocompatible scaffold material is very promising for tissue engineering.
It is shear thinning but has the downside of not being degradable in animals, it can only be degraded by cellulase enzymes.
In this study, a newly developed bioreactor was used to culture fibroblast spheroids under flow conditions inside nanocellulose hydrogels with and without the presence of cellulase.

Abstract
The aim was to control the tissue size and ideally find a match between degradation and tissue formation within this promising material.
Both the concentration of cellulase and the flow rate were varied and their influence on the activity and growth of fibroblast clusters was assessed.
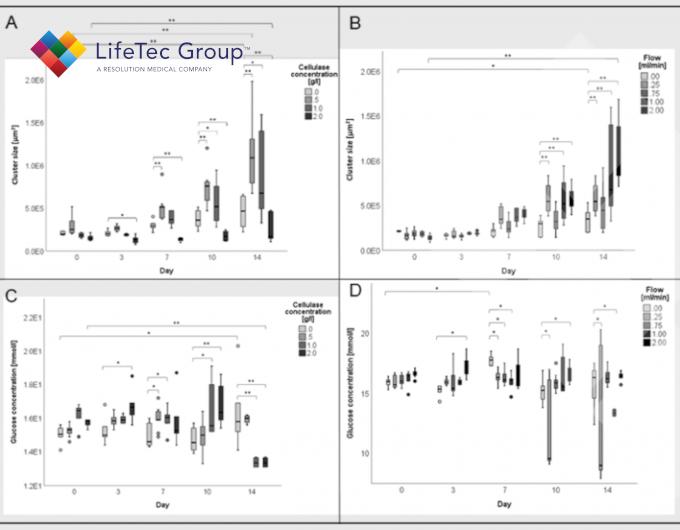
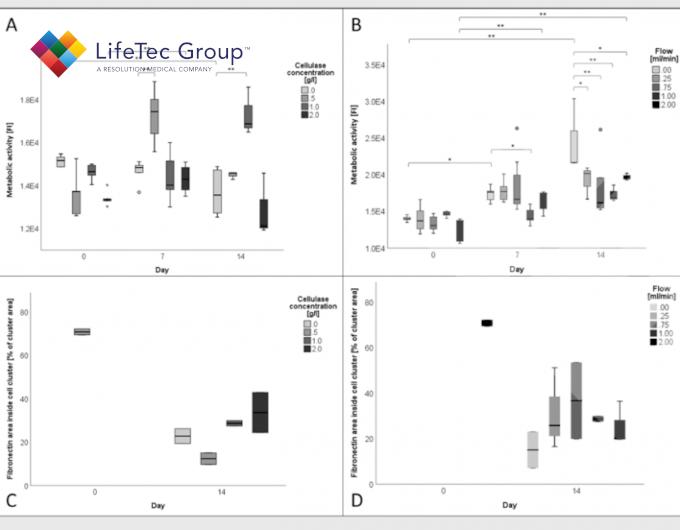
Cluster diameters, degradation, metabolic activity and tissue production increase with higher cellulase concentration, although concentrations above 1 g/l do not have additional benefit.
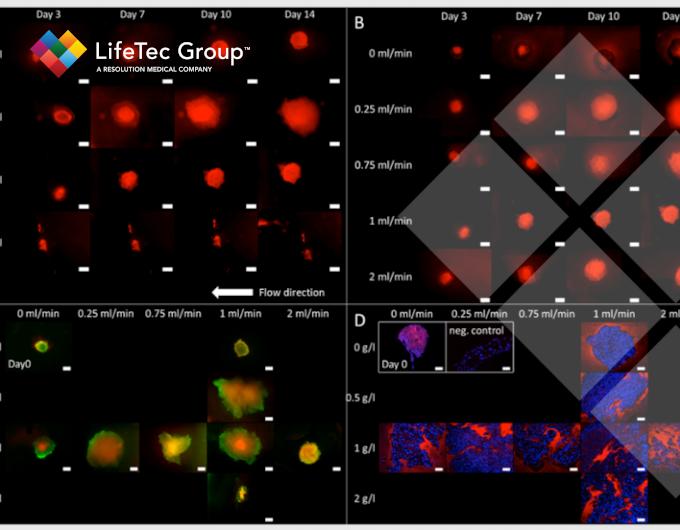
Flow leads to more viable cells, more proliferation and migration, leading to overall larger tissue constructs compared to static conditions. This is most likely due to the shear thinning effect of flow on cellulose nanofibrils in addition to the increased nutrient supply through perfusion.
At a constant cellulase concentration of 1 g/l, a flow of 2 ml/min proved to be to optimal for tissue production.
Therefore, degradation in combination with flow leads to more effective tissue production in cellulose nanofibril hydrogels, which is a very potent scaffold material for tissue engineering.
Effect of degradation by enzyme and/or flow on cluster sizes and glucose concentrations
Visualization of cluster growth, live/dead stainings, and fibronectin formation in clusters
Metabolic activity and quantification of fibronectin in cell clusters
Introduction
In tissue engineering, biomaterials replace native tissue partly or completely. The materials hereby serve as a 3D scaffold that permits maintenance of the cells and their signaling and allows for proliferation, differentiation and extracellular matrix (ECM) production to enable the development of new tissue with the desired function.
One obvious choice of scaffold material for this application are hydrogels due to their properties similar to tissue. Additionally, the development of bioreactors that create physiologically relevant culture conditions is essential for successful tissue engineering.
For the success in tissue engineering it is of utmost importance for in vitro tissues to receive sufficient nutrient and oxygen supply, therefore it is essential to either control the tissue size in a way to avoid dead cores or to provide means of perfusion into the tissue as for example by vascularization.
Cellulose has proven to be an emerging biomaterial in the field of cell culture. It is biocompatible and cell adhesive, has tunable mechanical properties, is shear-thinning, which is interesting for many scaffold fabrication techniques, and is not degradable without the addition of cellulase. Cellulose is widely available as it is the most abundant biopolymer on earth, which is directly isolated from wood and is mainly found in the cell walls of plants, where it is organized into highly crystalline microfibrils and can be chemically modified to form stable hydrogels.
These hydrogels have shown promising results in tissue engineering applications such as culturing of stem cells, 3D liver cell culture and as a sacrificial template for the engineering of tubular fibroblast constructs. Furthermore, cellulose nanofibrils (CNFs) exhibit stable functionality and fabrications costs are very low compared to other biomaterials.
As an interesting property, CNFs are not degradable within animals due to the lack of appropriate enzymes. Only the enzyme cellulase can degrade cellulose into glucose and it has been shown that this degradation and its degradation products do not harm animal cells in vitro. This means that cells can be cultured undisturbed on CNF substrates until confluency, after which the material can be degraded on demand by the addition of cellulase. However, to culture cells inside a CNF gel or enable cell infiltration in the body, gradual degradation is required.
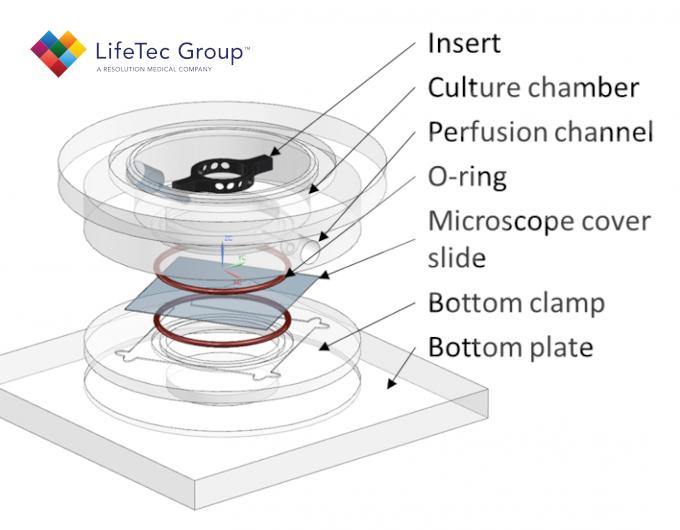
Therefore, in order to fully exploit the potential of CNF hydrogels as tissue engineering scaffolds it is essential to gain insight into how to induce and control their degradation kinetics. It is important that the rate of degradation matches the tissue formation, so that the mechanical support of the CNF scaffold persists as long as it is required. Here, we aim to achieve a better understanding of the conditions needed to grow cells inside 3D CNF hydrogels with the use of a custom-made bioreactor. Additional to enzymatic degradation, we hypothesize that fluid flow can aid degradation due to the shear thinning properties of the CNF hydrogel.
Publisher:
You can find the original paper @ ASME (publishers website)
Authors:
Melanie Krüger
LifeTec Group BV
Kennedyplein 10-11; 5611 ZS Eindhoven; the Netherlands
Universiteit Utrecht; Veterinary Medicine
Uppsalalaan 8; 3584 CT Utrecht; the Netherlands
Bart Spee
Universiteit Utrecht; Veterinary Medicine
Uppsalalaan 8; 3584 CT Utrecht; the Netherlands
Andreas Walther
Albert-Ludwigs-Universität Freiburg; Institute for Macromolecular Chemistry
Stefan-Meier-Strasse 3; Hermann Staudinger Building; 79104 Freiburg; Germany
andreas.walther@makro.uni-freiburg.de
Laura De Laporte
DWI – Leibniz-Institut für Interaktive Materialien e.V.; Advanced Materials for Biomedicine
ITMC – Institute of Technical and Macromolecular Chemistry; RWTH University AachenForckenbeckstr. 50; 52056 Aachen; Germany
Linda M. Kock
LifeTec Group BV
Kennedyplein 10-11; 5611 ZS Eindhoven; the Netherlands
The project leading to this publication has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie grant agreement No 64268.
- FUNDING
Horizon 2020

Interested in more about what we do at LifeTec Group? Contact us!
Call at +31 40 2989393 Or e-mail us
