Scientific paper:
"A novel hybrid silk-fibroin/polyurethane three-layered vascular graft: towards in situ tissue engineered vascular accesses for haemodialysis."
Keywords:
In situ tissue engineering, haemodialysis vascular access, electrospinning, early cannulation, hybrid vascular graft, fibroin, polyurethane
Introduction:
Patients undergoing chronic haemodialysis need a vascular access to connect the vascular system to an external dialysis equipment.
However, Clinically available alternatives of vascular access for long-term haemodialysis (currently limited to native arteriovenous fistulae and synthetic grafts) suffer from several drawbacks and are associated to high failure rates.
The best practice for recurrent long-term haemodialysis is the creation of an AVF, i.e., the direct surgical connection between an artery and a vein, which provides the highest long-term patency rates (approximately 70%) due to its capability of remodelling and evolving together with the patient’s system.
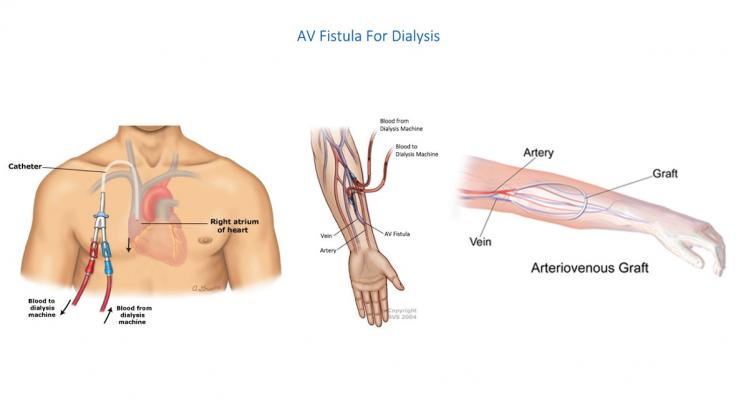
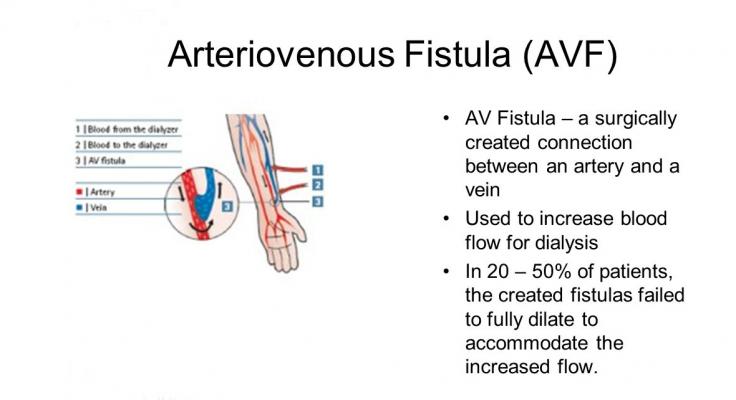
However, AVF is not always feasible in all patients (e.g., elderly, diabetics, patients with high Body Mass Index), and it usually requires 6-8 weeks to reach a sufficient maturation stage to allow for puncturing (process known as “arterialization”)
Abstract
Clinically available alternatives of vascular access for long-term haemodialysis(currently limited to native arteriovenous fistulae and synthetic grafts) suffer from several drawbacks and are associated to high failure rates.
Bioprosthetic grafts and tissue-engineered blood vessels are costly alternatives without clearly demonstrated increased performance.

"In situ tissue engineering could be the ideal approach"
In situ tissue engineering could be the ideal approach to provide a vascularaccess that profits from the advantages of vascular grafts in the short-term (e.g. early cannulation) and of fistulae in the long-term (e.g. high success rates driven by biointegration).
Hence, in this study a three-layered silk fibroin/polyurethane vascular graft was developed by electrospinning to be applied as long-term haemodialysisvascular access pursuing a 'hybrid' in situ engineering approach (i.e. based on a semi-degradable scaffold).
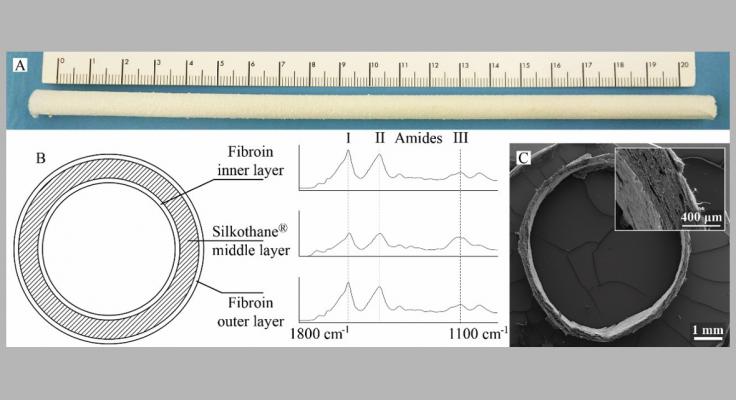
"three-layered silk fibroin/polyurethane vascular graft"
This Silkothane® graft was characterized concerning morphology, mechanics, physical properties, blood contact and vascular cell adhesion/viability. The full three-layered graft structure, influenced by the polyurethane presence, ensured mechanical properties that are a determinant factor for the success of a vascular access (e.g. vein-graft compliance matching).
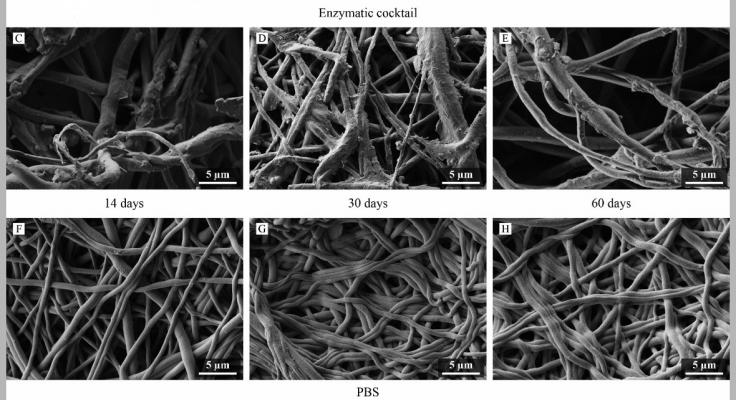
The Silkothane® graft demonstrated early cannulation potential in line with self-sealing commercial synthetic arteriovenous grafts, and a degradability driven by enzymatic activity.
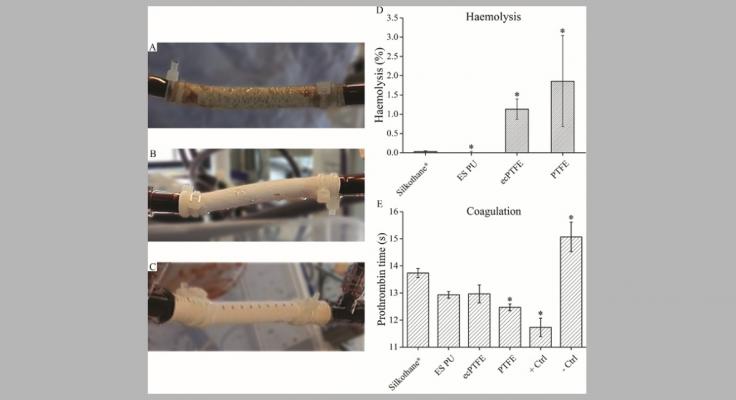
Moreover, the fibroin-only layers and extracellular matrix-like morphology, presented by the graft, revealed to be crucial in providing a non-haemolytic character, long clotting time, and favourable adhesion of human umbilical vein endothelial cells with increasing viability after 3 and 7 d.
Accordingly, the proposed approach may represent a step forward towards an in situ engineered hybrid vascular access with potentialities for vein-graft anastomosis stability, early cannulation, and biointegration.
Testimonials
Authors:
Sebastião van Uden, Noemi Vanerio, Valentina Catto, Barbara Bonandrini, Matteo Tironi, Marina Figliuzzi, Andrea Remuzzi, Linda Kock, Alberto C L Redaelli, Francesco G Greco, Stefania A. Riboldi
- Bioengineering Laboratories S.r.l., Cantù (MB), Italy
- Dipartimento di Elettronica Informazione e Bioingegneria, Politecnico di Milano, Milan (MI), Italy
- LifeTec Group BV, Eindhoven, The Netherlands
- Department of Cardiothoracic Surgery & Amsterdam Cardiovascular Sciences, Amsterdam University Medical Center, Amsterdam, The Netherlands
- Department of Chemistry, Materials and Chemical Engineering Giulio Natta, Politecnico di Milano, Milan (MI), Italy
- Istituto di Ricerche Farmacologiche Mario Negri IRCCS
- Dipartimento di Ingegneria gestionale, dell'informazione e della produzione, Università degli Studi di
Relevant links and background information:
- IOP Publishing Ltd [link]
- LifeTec Group - Vascular Bioreactor Platform [fact page]
- LifeTec Group - MUSICARE [case description page]
Interested in more about what we do at LifeTec Group? Contact us!
Call at +31 40 2989393 Or e-mail us

