Study / Scientific Publication
"An Innovative Ex Vivo Vascular Bioreactor as Comprehensive Tool to Study the Behavior of Native Blood Vessels Under Physiologically Relevant Conditions"
Keywords: Bioreactors, Blood vessels, Physiology, Shear stress, Ultrasonic imaging, Vessels, Biological tissues, Biology, Blood, Carbon dioxide, Carotid arteries, Drugs, Flow (Dynamics)
Abstract:
Ex vivo systems represent important models to study vascular biology and to test medical devices, combining the advantages of in vitro and in vivo models such as controllability of parameters and presence of biological response, respectively.
The aim of this study was to develop a comprehensive ex vivo vascular bioreactor to long-term culture and study the behavior of native blood vessels under physiologically relevant conditions.
The system, the experiment
The system was designed to allow for physiological mechanical loading in terms of pulsatile hemodynamics, shear stress and longitudinal pre-stretch and ultrasound imaging for vessel diameter and morphology evaluation.
In this first experience, porcine carotid arteries (n = 4) from slaughterhouse animals were cultured in the platform for 10 days at physiological temperature, CO2 and humidity using medium with blood-mimicking viscosity, components and stability of composition.
Vascular Bioreactor Platform
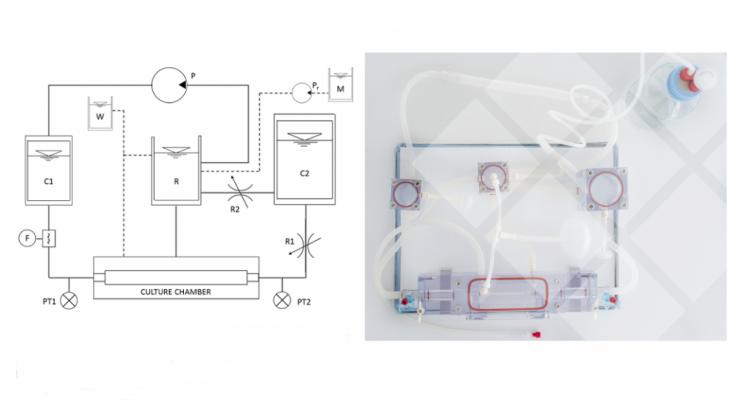
As expected, a significant increase in vessel diameter was observed during culture.
Flow rate was adjusted according to diameter values to reproduce and maintain physiological shear stress, while pressure was kept physiological.
Ultrasound imaging showed that the morphology and structure of cultured arteries were comparable to in vivo.
Histological analyses showed preserved endothelium and extracellular matrix and neointimal tissue growth over 10 days of culture.
In conclusion
In conclusion, we have developed a comprehensive pulsatile system in which a native blood vessel can be cultured under physiological conditions.
The present model represents a significant step towards ex vivo testing of vascular therapies, devices, drug interaction and as basis for further model developments.
Author and Article Information:
Noemi Vanerio
LifeTec Group BV, Kennedyplein 10-11, 5611 ZS, Eindhoven, The Netherlands;
Amsterdam University Medical Center, AMC - Department of cardio-thoracic surgery, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands
Marco Stijnen
LifeTec Group BV, Kennedyplein 10-11, 5611 ZS, Eindhoven, The Netherlands
Bas de Mol
Amsterdam University Medical Center, AMC - Department of cardio-thoracic surgery, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands
Linda M. Kock
LifeTec Group BV, Kennedyplein 10-11, 5611 ZS, Eindhoven, The Netherlands
Email: n.vanerio@lifetecgroup.com; n.vanerio@amc.uva.nl
Email: m.stijnen@lifetecgroup.com
Email: b.a.demol@amc.uva.nl
Email: l.kock@lifetecgroup.com
- - - - - - - - -
Paper No: JESMDT-19-1008 ( https://doi.org/10.1115/1.4044472 )
Published Online: August 1, 2019
Interested in more about what we do at LifeTec Group? Contact us!
Call at +31 40 2989393 Or e-mail us
